Established Blueprint for Development with First Mover Advantage
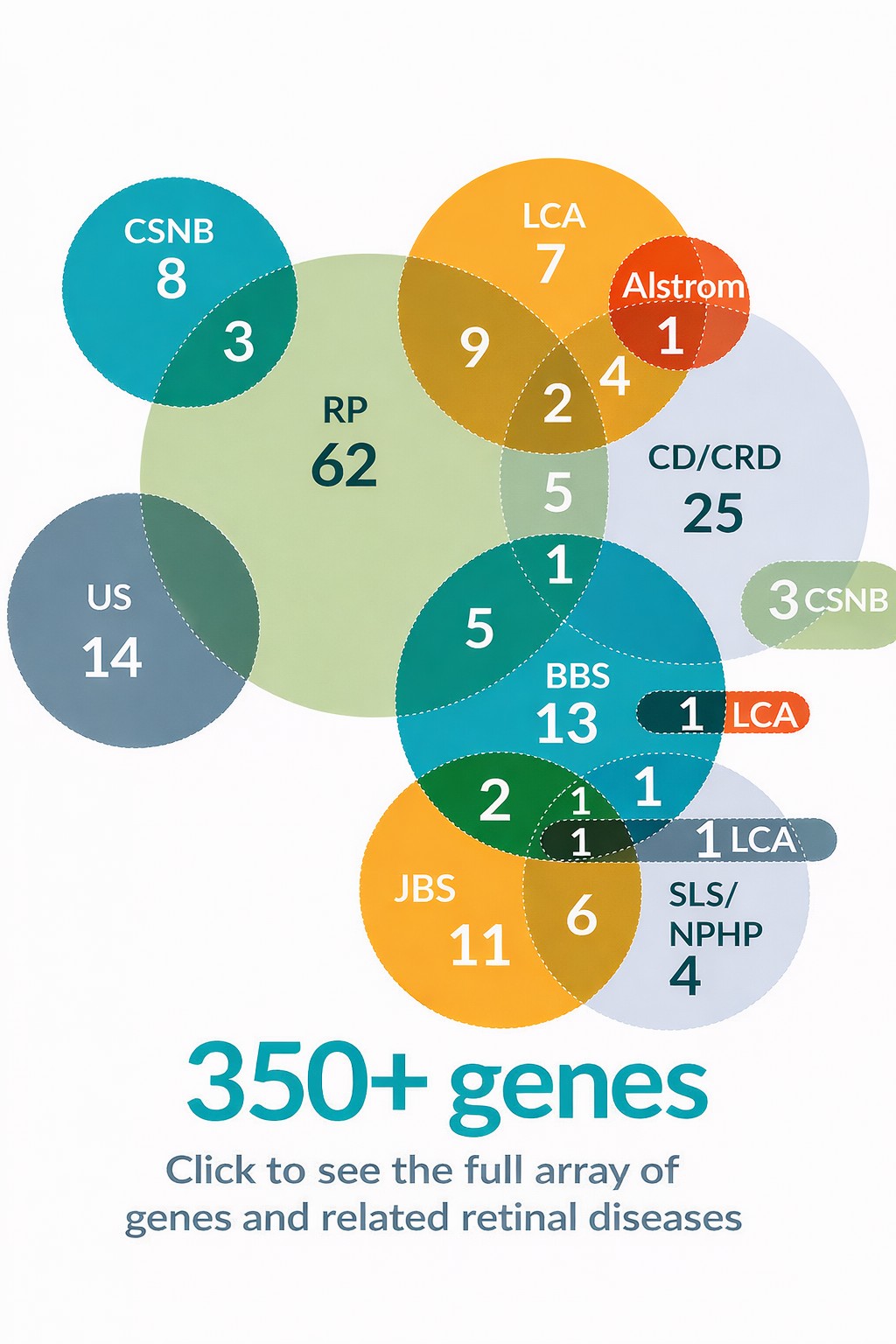
It’s estimated that over 300,000 patients are waiting for treatments for their individual genetic conditions, and more than 350 genes are known to cause inherited retinal diseases.
Opus Genetics’ mission is to pave a proven, derisked, efficient path to the clinic for urgently needed new therapies. Our IRD programs address mutations in genes that cause different forms of bestrophinopathy, Leber congenital amaurosis (LCA) and retinitis pigmentosa (RP), and are based on world-class science from gene therapy pioneers at the University of Pennsylvania (including the lab of Dr. Jean Bennett), Harvard Medical School, and the University of Florida.
Opus’ unique approach
- World-class science from pioneers in clinical gene therapy targeting high unmet need in multiple IRDs
- Rigorous selection of clinical programs grounded in natural history studies and patient registries
- Validation using large animal models
- Regulatory designations and significant funding received from the FDA, supporting innovative therapies
-
- Multi-million dollar grant from the FDA Office of Orphan Drug Products for clinical development
-
- Rare Pediatric Disease, Orphan Drug, Regenerative Medicine Advanced Therapy (RMAT) designations for lead program
“Although considered rare, vision-threatening inherited retinal diseases affect over 2 million people worldwide. Over 350 genes have been identified which are responsible for these conditions, but since 2017 only one gene therapy has been approved for a single class of IRD. We intend to address the need for therapies by developing a tailored manufacturing approach to support a sustainable pipeline validated by world-class scientists, to significantly expand the number of gene therapies available for retinal diseases.”
—Ben Yerxa, PhD, President, Opus Genetics