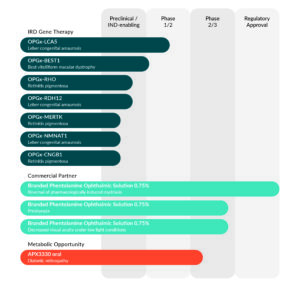
Strong and growing portfolio
Opus’ programs focus on treatments for inherited retinal diseases (IRDs), which address mutations in genes that cause loss of vision, including Leber congenital amaurosis (LCA), Best vitelliform macular dystrophy (BVMD), and retinitis pigmentosa (RP), as well as non-selective alpha-1 and alpha-2 adrenergic antagonist Phentolamine Ophthalmic Solution 0.75% and novel small-molecule inhibitor of Ref-1 APX3330.